- Record: found
- Abstract: found
- Article: found
Synapse-Selective Control of Cortical Maturation and Plasticity by Parvalbumin-Autonomous Action of SynCAM 1

Read this article at
SUMMARY
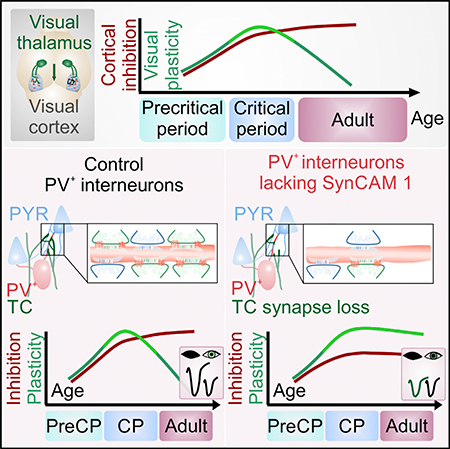
Cortical plasticity peaks early in life and tapers in adulthood, as exemplified in the primary visual cortex (V1), wherein brief loss of vision in one eye reduces cortical responses to inputs from that eye during the critical period but not in adulthood. The synaptic locus of cortical plasticity and the cellautonomous synaptic factors determining critical periods remain unclear. We here demonstrate that the immunoglobulin protein Synaptic Cell Adhesion Molecule 1 (SynCAM 1/Cadm1) is regulated by visual experience and limits V1 plasticity. Loss of SynCAM 1 selectively reduces the number of thalamocortical inputs onto parvalbumin (PV +) interneurons, impairing the maturation of feedforward inhibition in V1. SynCAM 1 acts in PV + interneurons to actively restrict cortical plasticity, and brief PV +-specific knockdown of SynCAM 1 in adult visual cortex restores juvenile-like plasticity. These results identify a synapse-specific, cell-autonomous mechanism for thalamocortical visual circuit maturation and closure of the visual critical period.
In Brief
Ribic et al. show that cortical plasticity is actively restricted by the synapseorganizing molecule SynCAM 1. The protein acts in parvalbumin interneurons to recruit excitatory thalamocortical terminals. This controls the maturation of inhibition and actively limits cortical plasticity, revealing a synaptic locus for closure of cortical critical periods.
Graphical Abstract
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Highly selective receptive fields in mouse visual cortex.
- Record: found
- Abstract: found
- Article: not found
Imaging large-scale neural activity with cellular resolution in awake, mobile mice.
- Record: found
- Abstract: found
- Article: not found