- Record: found
- Abstract: found
- Article: found
Lyme Disease, Virginia, USA, 2000–2011
Read this article at
Abstract
Geographic expansion of Ixodes scapularis ticks has increased human exposure to Borrelia burgdorferi.
Abstract
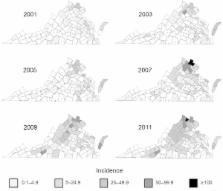
Lyme disease, caused by the bacterium Borrelia burgdorferi and transmitted in the eastern United States by the black-legged tick ( Ixodes scapularis), is increasing in incidence and expanding geographically. Recent environmental modeling based on extensive field collections of host-seeking I. scapularis ticks predicted a coastal distribution of ticks in mid-Atlantic states and an elevational limit of 510 m. However, human Lyme disease cases are increasing most dramatically at higher elevations in Virginia, a state where Lyme disease is rapidly emerging. Our goal was to explore the apparent incongruity, during 2000–2011, between human Lyme disease data and predicted and observed I. scapularis distribution. We found significantly higher densities of infected ticks at our highest elevation site than at lower elevation sites. We also found that I. scapularis ticks in Virginia are more closely related to northern than to southern tick populations. Clinicians and epidemiologists should be vigilant in light of the changing spatial distributions of risk.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe.
- Record: found
- Abstract: found
- Article: not found