- Record: found
- Abstract: found
- Article: found
Splenic CD4 + T Cells in Progressive Visceral Leishmaniasis Show a Mixed Effector-Regulatory Phenotype and Impair Macrophage Effector Function through Inhibitory Receptor Expression
Read this article at
Abstract
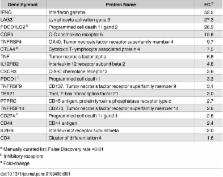
Visceral leishmaniasis (VL), caused by infection with the intracellular protozoan Leishmania donovani, is a chronic progressive disease with a relentlessly increasing parasite burden in the spleen, liver and bone marrow. The disease is characterized by fever, splenomegaly, cachexia, and pancytopenia, and progresses to death if not treated. Control of Leishmania infection is mediated by Th1 (IFNγ-producing) CD4 + T cells, which activate macrophages to produce nitric oxide and kill intracellular parasites. However, despite expansion of CD4 + T cells and increased IFNγ expression in the spleen, humans with active VL do not control the infection. We used an experimental model of chronic progressive VL in hamsters, which mimics clinical and pathological features seen in humans, to better understand the mechanisms that lead to progressive disease. Transcriptional profiling of the spleen during chronic infection revealed expression of markers of both T cell activation and inhibition. CD4 + T cells isolated from the spleen during chronic progressive VL showed mixed expression of Th1 and Th2 cytokines and chemokines, and were marginally effective in controlling infection in an ex vivo T cell-macrophage co-culture system. Splenic CD4 + T cells and macrophages from hamsters with VL showed increased expression of inhibitory receptors and their ligands, respectively. Blockade of the inhibitory receptor PD-L2 led to a significant decrease in parasite burden, revealing a pathogenic role for the PD-1 pathway in chronic VL. PD-L2 blockade was associated with a dramatic reduction in expression of host arginase 1, but no change in IFNγ and inducible nitric oxide synthase. Thus, the expression of counter-regulatory molecules on splenic CD4 + T cells and macrophages promotes a more permissive macrophage phenotype and attenuates intracellular parasite control in chronic progressive VL. Host-directed adjunctive therapy targeting the PD-1 regulatory pathway may be efficacious for VL.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity.
- Record: found
- Abstract: found
- Article: not found
Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection
- Record: found
- Abstract: found
- Article: not found