- Record: found
- Abstract: found
- Article: not found
L-Carnitine Preserves Endothelial Function in a Lamb Model of Increased Pulmonary Blood Flow
Read this article at
Abstract
Background
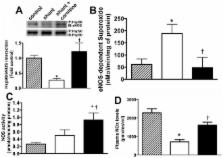
In our model of congenital heart disease (CHD) with increased pulmonary blood flow (Shunt), we have recently shown a disruption in carnitine homeostasis, associated with mitochondrial dysfunction and decreased eNOS/Hsp90 interactions that contribute to eNOS uncoupling, increased superoxide levels, and decreased bioavailable NO. Thus, we undertook this study to test the hypothesis that L-carnitine therapy would maintain mitochondrial function, and NO signaling.
Methods
Thirteen fetal lambs underwent in utero placement of an aortopulmonary graft. Immediately following delivery, lambs received daily treatment with oral L-carnitine or its vehicle.
Results
L-carnitine-treated lambs had decreased levels of acyl carnitine, and a reduced acyl carnitine: free carnitine ratio compared to vehicle treated Shunt lambs. These changes correlated with increased carnitine acetyl transferase (CrAT) protein and enzyme activity and decreased levels of nitrated CrAT. The lactate: pyruvate ratio was also decreased in L-carnitine-treated lambs. Hsp70 protein levels were significantly decreased and this correlated with increases in eNOS/Hsp90 interactions, NOS activity, NOx levels, and a significant decrease in eNOS-derived superoxide. Further, acetylcholine significantly decreased left pulmonary vascular resistance (PVR) only in L-carnitine-treated lambs.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Mitochondrial reactive oxygen species-mediated signaling in endothelial cells.
- Record: found
- Abstract: found
- Article: not found
Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders.
- Record: found
- Abstract: found
- Article: not found
