- Record: found
- Abstract: found
- Article: found
Targeting GLP-1 receptor trafficking to improve agonist efficacy
Read this article at
Abstract
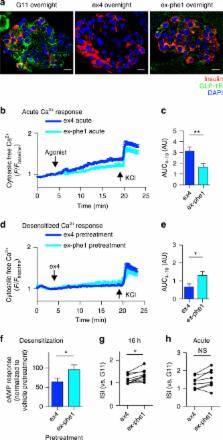
Glucagon-like peptide-1 receptor (GLP-1R) activation promotes insulin secretion from pancreatic beta cells, causes weight loss, and is an important pharmacological target in type 2 diabetes (T2D). Like other G protein-coupled receptors, the GLP-1R undergoes agonist-mediated endocytosis, but the functional and therapeutic consequences of modulating GLP-1R endocytic trafficking have not been clearly defined. Here, we investigate a series of biased GLP-1R agonists with variable propensities for GLP-1R internalization and recycling. Compared to a panel of FDA-approved GLP-1 mimetics, compounds that retain GLP-1R at the plasma membrane produce greater long-term insulin release, which is dependent on a reduction in β-arrestin recruitment and faster agonist dissociation rates. Such molecules elicit glycemic benefits in mice without concomitant increases in signs of nausea, a common side effect of GLP-1 therapies. Our study identifies a set of agents with specific GLP-1R trafficking profiles and the potential for greater efficacy and tolerability as T2D treatments.
Abstract
Glucagon-like peptide-1 receptor (GLP-1R) promotes insulin secretion from pancreatic beta cells and undergoes agonist-mediated endocytosis. Here, authors study GLP-1R endocytosis caused by different agonists and show that a longer plasma membrane retention time of GLP-1R results in greater long-term insulin release.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss.
- Record: found
- Abstract: found
- Article: not found
Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain.
- Record: found
- Abstract: found
- Article: found