- Record: found
- Abstract: found
- Article: found
Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): protocol for a multicentre randomised controlled trial
Read this article at
Abstract
Introduction
Intracytoplasmic sperm injection (ICSI), originally introduced as add-on to in vitro fertilisation (IVF) for couples with severe male infertility, is in current clinical practice also used in couples with mild male or even unexplained infertility. However, ICSI has involved unresolved concerns regarding the selection and damage to gametes and the health conditions of the offspring, and it is also labour intensive and therefore more expensive than conventional IVF. High-quality well-powered randomised clinical trials (RCTs) comparing ICSI and IVF are lacking.
Methods and analysis
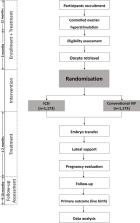
We propose a multicentre, open-label RCT in 10 reproductive medical centres across China. We will study couples with non-severe male infertility (defined as a semen concentrate 5–15×10 6/mL or sperm with a progressive motility 10%–32%) scheduled for their first or second ICSI or IVF cycle, as low fertility rate after fertilisation are more frequent in this population, which could lead to controversy about ICSI or conventional IVF for fertilisation. On the day of oocyte retrieval, eligible participants are after informed consent be randomised to undergo either ICSI or conventional IVF in a 1:1 treatment ratio. Other standard assisted reproductive treatments are similar and parallel between two groups. Our primary outcome is ongoing pregnancy leading to live birth after the first cycle with embryo transfer. To demonstrate or refute a difference of 7% between ICSI and conventional IVF, we need to include 2346 women (1173 in each intervention arm). In addition, we will follow-up neonatal outcomes after delivery to identify the influence of ICSI on offspring.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010.
- Record: found
- Abstract: not found
- Article: not found
Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte
- Record: found
- Abstract: not found
- Article: not found