- Record: found
- Abstract: found
- Article: found
Comparative Surface Morphology, Chemical Composition, and Cytocompatibility of Bio-C Repair, Biodentine, and ProRoot MTA on hDPCs
Read this article at
Abstract
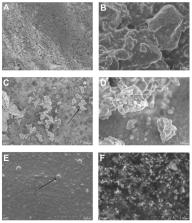
Biocompatibility is an essential property for any vital pulp material that may interact with the dental pulp tissues. Accordingly, this study aimed to compare the chemical composition and ultrastructural morphology of Biodentine (Septodont, Saint Maur-des-Fosses, France), ProRoot MTA (Dentsply Tulsa Dental Specialties, Johnson City, TN, USA), and Bio-C Repair (Angelus, Londrina, PR, Brazil), as well as their biological effects on human dental pulp cells. Chemical element characterization of the materials was undertaken using scanning electron microscopy and energy dispersive X-ray analysis (SEM-EDX). The cytotoxicity was assessed by analyzing the cell viability (MTT assay), cell morphology (immunofluorescence assay), and cell attachment (flow cytometry assay). The results were statistically analyzed using ANOVA and Tukey’s test ( p < 0.05). EDX revealed that ProRoot MTA and Biodentine were mostly composed of calcium, carbon, and oxygen (among others), whereas Bio-C Repair evidenced a low concentration of calcium and the highest concentration of zirconium. SEM showed adequate attachment of human dental pulp cells (hDPCS) to vital pulp materials and cytoskeletal alterations were not observed in the presence of material eluates. Remarkably, the undiluted Biodentine group showed higher viability than the control group cells (without eluates) at 24 h, 48 h, and 72 h ( p < 0.001). Based on the evidence derived from an in vitro cellular study, it was concluded that Bio-C Repair showed excellent cytocompatibility that was similar to Biodentine and ProRoot MTA.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Physicochemical basis of the biologic properties of mineral trioxide aggregate.
- Record: found
- Abstract: found
- Article: not found
Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus.
- Record: found
- Abstract: found
- Article: not found