- Record: found
- Abstract: found
- Article: found
Increased S100A4 expression in the vasculature of human COPD lungs and murine model of smoke-induced emphysema
Read this article at
Abstract
Background
Chronic obstructive lung disease (COPD) is a common cause of death in industrialized countries often induced by exposure to tobacco smoke. A substantial number of patients with COPD also suffer from pulmonary hypertension that may be caused by hypoxia or other hypoxia-independent stimuli - inducing pulmonary vascular remodeling. The Ca 2+ binding protein, S100A4 is known to play a role in non-COPD-driven vascular remodeling of intrapulmonary arteries. Therefore, we have investigated the potential involvement of S100A4 in COPD induced vascular remodeling.
Methods
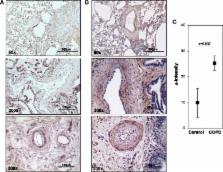
Lung tissue was obtained from explanted lungs of five COPD patients and five non-transplanted donor lungs. Additionally, mice lungs of a tobacco-smoke-induced lung emphysema model (exposure for 3 and 8 month) and controls were investigated. Real-time RT-PCR analysis of S100A4 and RAGE mRNA was performed from laser-microdissected intrapulmonary arteries. S100A4 immunohistochemistry was semi-quantitatively evaluated. Mobility shift assay and siRNA knock-down were used to prove hypoxia responsive elements (HRE) and HIF binding within the S100A4 promoter.
Results
Laser-microdissection in combination with real-time PCR analysis revealed higher expression of S100A4 mRNA in intrapulmonary arteries of COPD patients compared to donors. These findings were mirrored by semi-quantitative analysis of S100A4 immunostaining. Analogous to human lungs, in mice with tobacco-smoke-induced emphysema an up-regulation of S100A4 mRNA and protein was observed in intrapulmonary arteries. Putative HREs could be identified in the promoter region of the human S100A4 gene and their functionality was confirmed by mobility shift assay. Knock-down of HIF1/2 by siRNA attenuated hypoxia-dependent increase in S100A4 mRNA levels in human primary pulmonary artery smooth muscle cells. Interestingly, RAGE mRNA expression was enhanced in pulmonary arteries of tobacco-smoke exposed mice but not in pulmonary arteries of COPD patients.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
HIF-1: mediator of physiological and pathophysiological responses to hypoxia.
- Record: found
- Abstract: found
- Article: not found
Animal models of chronic obstructive pulmonary disease.
- Record: found
- Abstract: found
- Article: not found