- Record: found
- Abstract: found
- Article: found
Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders
Read this article at
Abstract
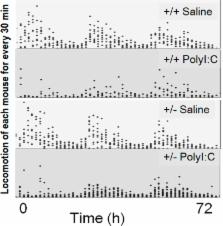
CB2 cannabinoid receptor (CB2R) gene is associated with depression. We investigated the gene-environment interaction between CB2R function and diverse stressors. First, anxiety-like behavior during chronic-mild-stress (CMS) was evaluated in C57BL/6JJmsSlc mice following treatment with CB2R agonist JWH015 or inverse-agonist AM630. Second, locomotor activity and anxiety-like behavior were measured following exposure to an immune poly I:C stressor. Gene expressions of HPA axis related molecules, Fkbp5, Nr3c1 and Crf and pro-inflammatory cytokine Il-1b, as well as Bdnf as a key neurotrophin that supports neuron health, function, and synaptic plasticity, were determined in hippocampus of Cnr2 knockout mice, as indicators of stressful environment. CMS-induced anxiety-like behavior was enhanced by AM630 and reduced by JWH015 and fluvoxamine. Poly I:C reduced locomotor activity and increased anxiety-like behavior, and these effects were pronounced in the heterozygote than in the wild type mice. Fkbp5 and Nr3c1 expression were lower in the Cnr2 heterozygotes than in the wild type mice with Poly I:C treatment. These findings indicate that interaction between CB2R gene and stressors increases the risk of depression-like behaviors that may be linked with neuro-immune crosstalk. Further studies in human subjects are necessary to determine the role of CB2R and environmental interaction in the development of depression.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Neurobiological Interactions Between Stress and the Endocannabinoid System.
- Record: found
- Abstract: found
- Article: not found
The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior.
- Record: found
- Abstract: found
- Article: not found