- Record: found
- Abstract: found
- Article: found
Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma
Read this article at
Abstract
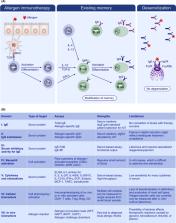
Modern health care requires a proactive and individualized response to diseases, combining precision diagnosis and personalized treatment. Accordingly, the approach to patients with allergic diseases encompasses novel developments in the area of personalized medicine, disease phenotyping and endotyping, and the development and application of reliable biomarkers. A detailed clinical history and physical examination followed by the detection of IgE immunoreactivity against specific allergens still represents the state of the art. However, nowadays, further emphasis focuses on the optimization of diagnostic and therapeutic standards and a large number of studies have been investigating the biomarkers of allergic diseases, including asthma, atopic dermatitis, allergic rhinitis, food allergy, urticaria and anaphylaxis. Various biomarkers have been developed by omics technologies, some of which lead to a better classification of distinct phenotypes or endotypes. The introduction of biologicals to clinical practice increases the need for biomarkers for patient selection, prediction of outcomes and monitoring, to allow for an adequate choice of the duration of these costly and long‐lasting therapies. Escalating healthcare costs together with questions about the efficacy of the current management of allergic diseases require further development of a biomarker‐driven approach. Here, we review biomarkers in diagnosis and treatment of asthma, atopic dermatitis, allergic rhinitis, viral infections, chronic rhinosinusitis, food allergy, drug hypersensitivity and allergen immunotherapy with a special emphasis on specific IgE, the microbiome and the epithelial barrier. In addition, EAACI guidelines on biologicals are discussed within the perspective of biomarkers.
Related collections
Most cited references352
- Record: found
- Abstract: not found
- Article: not found
GRADE: an emerging consensus on rating quality of evidence and strength of recommendations.
- Record: found
- Abstract: found
- Article: not found
GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables.
- Record: found
- Abstract: found
- Article: not found