- Record: found
- Abstract: found
- Article: found
A neuronal activation correlate in striatum and prefrontal cortex of prolonged cocaine intake
Read this article at
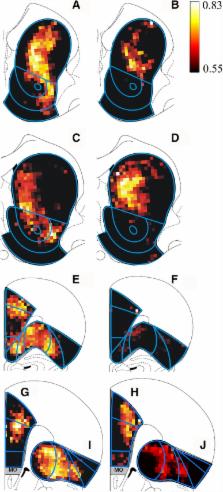
Abstract
Maladaptive changes in the involvement of striatal and frontal cortical regions in drug use are thought to underlie the progression to habitual drug use and loss of cognitive control over drug intake that occur with accumulating drug experience. The present experiments focus on changes in neuronal activity in these regions associated with short-term (10 days) and long-term (60 days) self-administration of cocaine. Quantitative in situ hybridization for the immediate early gene Mkp1 was combined with statistical parametric mapping to assess the distribution of neuronal activity. We hypothesized that neuronal activity in striatum would increase in its dorsal part and that activity in frontal cortex would decrease with prolonged cocaine self-administration experience. Expression of Mkp1 was profoundly increased after cocaine self-administration, and the magnitude of this effect was greater after short-term compared to long-term self-administration. Increased neuronal activity was seen in both dorsal and ventral sectors of the striatum after 10 days exposure to cocaine. However, enhanced activity was restricted to dorsomedial and dorsocentral striatum after 60 days cocaine self-administration. In virtually all medial prefrontal and most orbitofrontal areas, increased expression of Mkp1 was observed after 10 days of cocaine taking, whereas after 60 days, enhanced expression was restricted to caudal parts of medial prefrontal and caudomedial parts of orbitofrontal cortex. Our data reveal functional changes in cellular activity in striatum and frontal cortex with increasing cocaine self-administration experience. These changes might reflect the neural processes that underlie the descent from recreational drug taking to compulsive cocaine use.
Related collections
Most cited references101
- Record: found
- Abstract: not found
- Article: not found
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing
- Record: found
- Abstract: found
- Article: not found
Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications.
- Record: found
- Abstract: found
- Article: not found