- Record: found
- Abstract: found
- Article: found
Xanthones of Lichen Source: A 2016 Update
Read this article at
Abstract
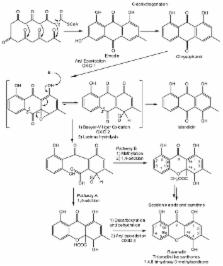
An update of xanthones encountered in lichens is proposed as more than 20 new xanthones have been described since the publication of the compendium of lichen metabolites by Huneck and Yoshimura in 1996. The last decades witnessed major advances regarding the elucidation of biosynthetic schemes leading to these fascinating compounds, accounting for the unique substitution patterns of a very vast majority of lichen xanthones. Besides a comprehensive analysis of the structures of xanthones described in lichens, their bioactivities and the emerging analytical strategies used to pinpoint them within lichens are presented here together with physico-chemical properties (including NMR data) as reported since 1996.
Related collections
Most cited references120
- Record: found
- Abstract: found
- Article: not found
Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi.
- Record: found
- Abstract: found
- Article: not found
Bacterial communities associated with the lichen symbiosis.
- Record: found
- Abstract: found
- Article: not found