- Record: found
- Abstract: found
- Article: found
Risk of adverse reactions associated with inhaled corticosteroids for chronic obstructive pulmonary disease: A meta-analysis
Read this article at
Abstract
Background:
In the majority of current therapeutic regimens for chronic obstructive pulmonary disease (COPD), bronchodilators are coupled with inhaled corticosteroids (ICS) to lower the inflammatory response and improve symptoms. This study aims to evaluate the safety of ICS in the treatment of COPD.
Methods:
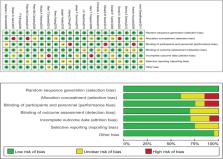
Randomized controlled trials related to ICS for COPD that were eligible up to 1 June 2023 were searched in PubMed, EMBASE, and Cochrane. We searched and screened eligible studies for the occurrence of total adverse events, cardiovascular events, upper respiratory tract infections (URTI), pneumonia, oral Candida infections, and musculoskeletal disorders, and finally analyzed them by Review Manager 5.4.1.
Results:
The results showed that ICS increased the incidence of adverse reactions in COPD patients (RR = 1.06, 95% CI: 1.03–1.10, P = .0004); ICS treatment did not increase the risk of cardiovascular events in COPD patients (RR = 0.95, 95% CI: 0.88–1.02, P = .14); ICS increased the incidence of URTI in COPD patients (RR = 1.29, 95% CI: 1.02–1.62, P = .03); ICS increased the incidence of pneumonia in patients with COPD (RR = 2.09, 95% CI: 1.63–2.69, P < .00001); ICS treatment significantly increased the incidence of oral Candida in patients with COPD (RR = 2.96, 95% CI: 1.99–4.41, P < .00001); ICS increased the incidence of musculoskeletal disorders in patients with COPD (RR = 2.87, 95% CI: 1.51–5.45, P = .001).
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Assessing the quality of reports of randomized clinical trials: is blinding necessary?
- Record: found
- Abstract: found
- Article: not found
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary
- Record: found
- Abstract: found
- Article: not found
