- Record: found
- Abstract: found
- Article: not found
Current perspectives of the signaling pathways directing neural crest induction
Read this article at
Abstract
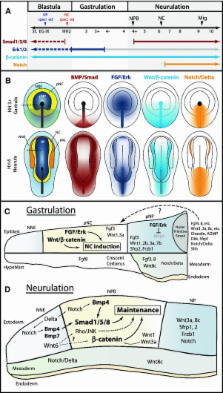
The neural crest is a migratory population of embryonic cells with a tremendous potential to differentiate and contribute to nearly every organ system in the adult body. Over the past two decades, an incredible amount of research has given us a reasonable understanding of how these cells are generated. Neural crest induction involves the combinatorial input of multiple signaling pathways and transcription factors, and is thought to occur in two phases from gastrulation to neurulation. In the first phase, FGF and Wnt signaling induce NC progenitors at the border of the neural plate, activating the expression of members of the Msx, Pax, and Zic families, among others. In the second phase, BMP, Wnt, and Notch signaling maintain these progenitors and bring about the expression of definitive NC markers including Snail2, FoxD3, and Sox9/10. In recent years, additional signaling molecules and modulators of these pathways have been uncovered, creating an increasingly complex regulatory network. In this work, we provide a comprehensive review of the major signaling pathways that participate in neural crest induction, with a focus on recent developments and current perspectives. We provide a simplified model of early neural crest development and stress similarities and differences between four major model organisms: Xenopus, chick, zebrafish, and mouse.
Related collections
Most cited references177
- Record: found
- Abstract: found
- Article: not found
FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment.
- Record: found
- Abstract: found
- Article: not found
LDL-receptor-related proteins in Wnt signal transduction.
- Record: found
- Abstract: found
- Article: not found
