- Record: found
- Abstract: found
- Article: found
Warburg effect in chemosensitivity: Targeting lactate dehydrogenase-A re-sensitizes Taxol-resistant cancer cells to Taxol
Read this article at
Abstract
Background
Taxol is one of the most effective chemotherapeutic agents for the treatment of patients with breast cancer. Despite impressive clinical responses initially, the majority of patients eventually develop resistance to Taxol. Lactate dehydrogenase-A (LDH-A) is one of the predominant isoforms of LDH expressed in breast tissue, which controls the conversion of pyruvate to lactate and plays an important role in glucose metabolism. In this study we investigated the role of LDH-A in mediating Taxol resistance in human breast cancer cells.
Results
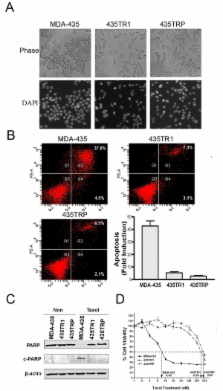
Taxol-resistant subclones, derived from the cancer cell line MDA-MB-435, sustained continuous growth in high concentrations of Taxol while the Taxol-sensitive cells could not. The increased expression and activity of LDH-A were detected in Taxol-resistant cells when compared with their parental cells. The downregulation of LDH-A by siRNA significantly increased the sensitivity of Taxol-resistant cells to Taxol. A higher sensitivity to the specific LDH inhibitor, oxamate, was found in the Taxol-resistant cells. Furthermore, treating cells with the combination of Taxol and oxamate showed a synergistical inhibitory effect on Taxol-resistant breast cancer cells by promoting apoptosis in these cells.
Conclusion
LDH-A plays an important role in Taxol resistance and inhibition of LDH-A re-sensitizes Taxol-resistant cells to Taxol. This supports that Warburg effect is a property of Taxol resistant cancer cells and may play an important role in the development of Taxol resistance. To our knowledge, this is the first report showing that the increased expression of LDH-A plays an important role in Taxol resistance of human breast cancer cells. This study provides valuable information for the future development and use of targeted therapies, such as oxamate, for the treatment of patients with Taxol-resistant breast cancer.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Cancer's molecular sweet tooth and the Warburg effect.
- Record: found
- Abstract: found
- Article: not found
Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis.
- Record: found
- Abstract: found
- Article: not found