- Record: found
- Abstract: found
- Article: found
Concise Review: Stem/Progenitor Cell Proteoglycans Decorated with 7‐D‐4, 4‐C‐3, and 3‐B‐3(‐) Chondroitin Sulfate Motifs Are Morphogenetic Markers of Tissue Development

Read this article at
Abstract
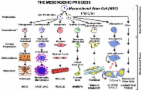
This study reviewed the occurrence of chondroitin sulfate (CS) motifs 4‐C‐3, 7‐D‐4, and 3‐B‐3(‐), which are expressed by progenitor cells in tissues undergoing morphogenesis. These motifs have a transient early expression pattern during tissue development and also appear in mature tissues during pathological remodeling and attempted repair processes by activated adult stem cells. The CS motifs are information and recognition modules, which may regulate cellular behavior and delineate stem cell niches in developmental tissues. One of the difficulties in determining the precise role of stem cells in tissue development and repair processes is their short engraftment period and the lack of specific markers, which differentiate the activated stem cell lineages from the resident cells. The CS sulfation motifs 7‐D‐4, 4‐C‐3, and 3‐B‐3 (‐) decorate cell surface proteoglycans on activated stem/progenitor cells and appear to identify these cells in transitional areas of tissue development and in tissue repair and may be applicable to determining a more precise role for stem cells in tissue morphogenesis. stem cells 2018;36:1475–1486
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Convergence of Wnt, beta-catenin, and cadherin pathways.
- Record: found
- Abstract: found
- Article: not found
The surface of articular cartilage contains a progenitor cell population.