- Record: found
- Abstract: found
- Article: found
Insights Into the Immune Response of the Black Soldier Fly Larvae to Bacteria
Read this article at
Abstract
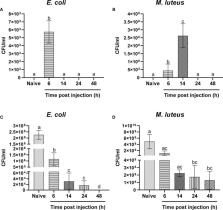
In insects, a complex and effective immune system that can be rapidly activated by a plethora of stimuli has evolved. Although the main cellular and humoral mechanisms and their activation pathways are highly conserved across insects, the timing and the efficacy of triggered immune responses can differ among different species. In this scenario, an insect deserving particular attention is the black soldier fly (BSF), Hermetia illucens (Diptera: Stratiomyidae). Indeed, BSF larvae can be reared on a wide range of decaying organic substrates and, thanks to their high protein and lipid content, they represent a valuable source of macromolecules useful for different applications (e.g., production of feedstuff, bioplastics, and biodiesel), thus contributing to the development of circular economy supply chains for waste valorization. However, decaying substrates bring the larvae into contact with different potential pathogens that can challenge their health status and growth. Although these life strategies have presumably contributed to shape the evolution of a sophisticated and efficient immune system in this dipteran, knowledge about its functional features is still fragmentary. In the present study, we investigated the processes underpinning the immune response to bacteria in H. illucens larvae and characterized their reaction times. Our data demonstrate that the cellular and humoral responses in this insect show different kinetics: phagocytosis and encapsulation are rapidly triggered after the immune challenge, while the humoral components intervene later. Moreover, although both Gram-positive and Gram-negative bacteria are completely removed from the insect body within a few hours after injection, Gram-positive bacteria persist in the hemolymph longer than do Gram-negative bacteria. Finally, the activity of two key actors of the humoral response, i.e., lysozyme and phenoloxidase, show unusual dynamics as compared to other insects. This study represents the first detailed characterization of the immune response to bacteria of H. illucens larvae, expanding knowledge on the defense mechanisms of this insect among Diptera. This information is a prerequisite to manipulating the larval immune response by nutritional and environmental factors to increase resistance to pathogens and optimize health status during mass rearing.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
Immune pathways and defence mechanisms in honey bees Apis mellifera

- Record: found
- Abstract: found
- Article: found
From bacterial killing to immune modulation: Recent insights into the functions of lysozyme
- Record: found
- Abstract: found
- Article: not found