- Record: found
- Abstract: found
- Article: found
The effect of atorvastatin on inflammatory markers in sulfur mustard gas induced bronchitis: a randomized double-blinded, placebo-control clinical trial
Read this article at
Abstract
Background
This study was performed to evaluate the anti-inflammatory effect of atorvastatin in patients with chronic bronchitis, exposed to sulfur mustard gas.
Methods
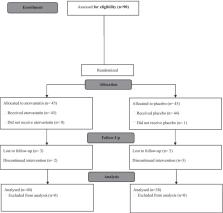
In this randomized double-blinded clinical trial we recruited patients with chronic bronchitis after exposure to sulfur mustard gas. Ninety men 45–75 years old diagnosed with chronic bronchitis after exposure to mustard gas during the Iran-Iraq war, were randomly assigned to receive either atorvastatin (40 mg) or placebo once a day for 3 months. The interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), procalcitonin, highly sensitive CRP and COPD assessment test (CAT) score was compared at baseline and after 12 weeks.
Results
After consuming atorvastatin for 12 weeks, IL-6 level (mean difference [95%CI]; 0.2 [− 0.05, 0.5]), TNF-α (mean difference [95%CI]; − 0.07 [− 0.2, 0.07]), high sensitive CRP (mean difference [95%CI] − 0.1 [− 1.2, 0.9]), and procalcitonin (mean difference [95%CI]; 0.003 [− 0.02, 0.03]) did not change significantly. However, in the placebo group, only IL-6 (mean difference [95%CI]; 0.6 [0.2, 1.05]) decreased significantly after 12 weeks, but levels of high sensitive CRP (mean difference [95%CI]; − 0.3 [− 1.4, 0.8]) TNF-α (mean difference [95%CI]; − 0.2 [− 0.34, − 0.06]) and procalcitonin (mean difference [95%CI]; 0.02 [− 0.001, 0.04]) did not change significantly. After 12 weeks, the mean differences in TNF- α, IL-6 level, high sensitive CRP, procalcitonin, and CAT score did not significantly differ between the two groups.
Conclusions
The administration of 40 mg atorvastatin for 3 months did not significantly change the inflammatory markers or the quality of life of patients exposed to mustard gas with chronic bronchitis.
Trial registration: IRCT, IRCT138904144312N1. Registered 16 August 2014, https://en.irct.ir/trial/4577.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences
- Record: found
- Abstract: found
- Article: not found
Development and first validation of the COPD Assessment Test.
- Record: found
- Abstract: found
- Article: not found