- Record: found
- Abstract: found
- Article: found
Establishment of CMab-43, a Sensitive and Specific Anti-CD133 Monoclonal Antibody, for Immunohistochemistry
Read this article at
Abstract
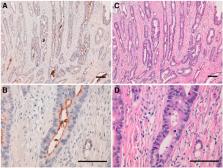
CD133, also known as prominin-1, was first described as a cell surface marker on early progenitor and hematopoietic stem cells. It is a five-domain transmembrane protein composed of an N-terminal extracellular tail, two small cytoplasmic loops, two large extracellular loops containing seven potential glycosylation sites, and a short C-terminal intracellular tail. CD133 has been used as a marker to identify cancer stem cells derived from primary solid tumors and as a prognostic marker of gliomas. Herein, we developed novel anti-CD133 monoclonal antibodies (mAbs) and characterized their efficacy in flow cytometry, Western blot, and immunohistochemical analyses. We expressed the full length of CD133 in LN229 glioblastoma cells, immunized mice with LN229/CD133 cells, and performed the first screening using flow cytometry. After limiting dilution, we established 100 anti-CD133 mAbs, reacting with LN229/CD133 cells but not with LN229 cells. Subsequently, we performed the second and third screening with Western blot and immunohistochemical analyses, respectively. Among 100 mAbs, 11 strongly reacted with CD133 in Western blot analysis. One of 11 clones, CMab-43 (IgG 2a, kappa), showed a sensitive and specific reaction against colon cancer cells, warranting the use of CMab-43 in detecting CD133 in pathological analyses of CD133-expressing cancers.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles.
- Record: found
- Abstract: found
- Article: not found
Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma.
- Record: found
- Abstract: found
- Article: not found