- Record: found
- Abstract: found
- Article: found
Improving rehabilitation in sarcopenia: a randomized‐controlled trial utilizing a muscle‐targeted food for special medical purposes
Read this article at
Abstract
Background
Sarcopenia is a disease associated with aging and a negative prognosis. Consensus‐based treatment consists in targeting muscle mass and function through physical exercise, optimization of protein intake, and vitamin D supplementation, but evidence is lacking. We evaluated the safety and efficacy of a muscle‐targeted nutritional support on the outcome of a physical exercise rehabilitation programme.
Methods
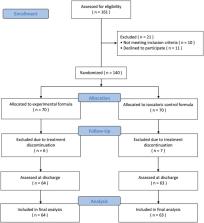
In a single‐site, double‐blind, randomized, controlled trial (NCT03120026; May 2017 to December 2018), old (≥65 years) adults [ N = 140 (63% female patients; age, 81 ± 6 years)] without severe cognitive impairment, who were found to have sarcopenia by European Working Group on Sarcopenia in Older People 2010 criteria and hospitalized for physical rehabilitation, were randomized to receive until discharge (for at least 4 weeks and up to 8 weeks) a whey protein‐based nutritional formula enriched with leucine and vitamin D or an iso‐caloric control formula twice daily in addition to a standard hospital diet. The primary endpoint was the change in 4 m gait speed per month. Key secondary endpoints addressed the change in physical performance: chair‐stand test, timed up and go test, and short physical performance battery. Other secondary outcomes were the change in functional status, muscle strength and mass, cognitive status, and quality of life. The proportion of patients who improved their rehabilitation intensity profile and overall economic benefits (using length of stay and duration of rehabilitation as surrogate measures) were also evaluated.
Results
A total of 161 patients were screened and 140 were randomized to study interventions. Thirteen patients (experimental, n = 6; placebo, n = 7) discontinued the intervention because they disliked the product and intention‐to‐treat analyses were based on patients reassessed at discharge [ n = 127 (66% female patients; age, 81 ± 6 years)]. Supplementation with the experimental formula ( n = 64) resulted in greater increase in mean gait speed {0.061 m/s/month [95% confidence interval (CI), 0.043 to 0.080]} than placebo [ n = 63; −0.001 m/s/month (95%CI, −0.008 to 0.006)]: mean difference, 0.063 m/s/month (95%CI, 0.043 to 0.082) ( P < 0.001). A significant effect was also found for muscle mass ( P < 0.03) and all key secondary outcomes, functional and cognitive endpoints ( P < 0.001 for all). Supplementation resulted also in higher proportion of patients improving their rehabilitation intensity profile ( P = 0.003) and being discharged home ( P = 0.002); shorter rehabilitation ( P < 0.001); and hospital stay ( P < 0.001).
Related collections
Most cited references56

- Record: found
- Abstract: found
- Article: found
Sarcopenia: revised European consensus on definition and diagnosis
- Record: found
- Abstract: found
- Article: not found