- Record: found
- Abstract: found
- Article: found
Complement System and Alarmin HMGB1 Crosstalk: For Better or Worse
Read this article at
Abstract
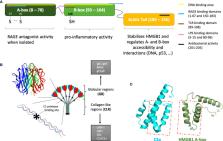
Our immune system responds to infectious (PAMPs) and tissue damage (DAMPs) signals. The complement system and alarmin High-Mobility Group Box 1 (HMGB1) are two powerful soluble actors of human host defense and immune surveillance. These systems involve molecular cascades and amplification loops for their signaling or activation. Initially activated as alarm raising systems, their function can be finally switched towards inflammation resolution, where they sustain immune maturation and orchestrate repair mechanisms, opening the way back to homeostasis. However, when getting out of control, these defense systems can become deleterious and trigger serious cellular and tissue damage. Therefore, they can be considered as double-edged swords. The close interaction between the complement and HMGB1 pathways is described here, as well as their traditional and non-canonical roles, their functioning at different locations and their independent and collective impact in different systems both in health and disease. Starting from these systems and interplay at the molecular level (when elucidated), we then provide disease examples to better illustrate the signs and consequences of their roles and interaction, highlighting their importance and possible vicious circles in alarm raising and inflammation, both individually or in combination. Although this integrated view may open new therapeutic strategies, future challenges have to be faced because of the remaining unknowns regarding the molecular mechanisms underlying the fragile molecular balance which can drift towards disease or return to homeostasis, as briefly discussed at the end.
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
HMG-1 as a late mediator of endotoxin lethality in mice.
- Record: found
- Abstract: found
- Article: not found
Complement: a key system for immune surveillance and homeostasis.

- Record: found
- Abstract: found
- Article: found