- Record: found
- Abstract: found
- Article: found
Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies
Read this article at
Abstract
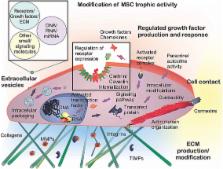
Adult mesenchymal stem cells (MSCs) represent a subject of intense experimental and biomedical interest. Recently, trophic activities of MSCs have become the topic of a number of revealing studies that span both basic and clinical fields. In this review, we focus on recent investigations that have elucidated trophic mechanisms and shed light on MSC clinical efficacy relevant to musculoskeletal applications. Innate differences due to MSC sourcing may play a role in the clinical utility of isolated MSCs. Pain management, osteochondral, nerve, or blood vessel support by MSCs derived from both autologous and allogeneic sources have been examined. Recent mechanistic insights into the trophic activities of these cells point to ultimate regulation by nitric oxide, nuclear factor-kB, and indoleamine, among other signaling pathways. Classic growth factors and cytokines—such as VEGF, CNTF, GDNF, TGF-β, interleukins (IL-1β, IL-6, and IL-8), and C-C ligands (CCL-2, CCL-5, and CCL-23)—serve as paracrine control molecules secreted or packaged into extracellular vesicles, or exosomes, by MSCs. Recent studies have also implicated signaling by microRNAs contained in MSC-derived exosomes. The response of target cells is further regulated by their microenvironment, involving the extracellular matrix, which may be modified by MSC-produced matrix metalloproteinases (MMPs) and tissue inhibitor of MMPs. Trophic activities of MSCs, either resident or introduced exogenously, are thus intricately controlled, and may be further fine-tuned via implant material modifications. MSCs are actively being investigated for the repair and regeneration of both osteochondral and other musculoskeletal tissues, such as tendon/ligament and meniscus. Future rational and effective MSC-based musculoskeletal therapies will benefit from better mechanistic understanding of MSC trophic activities, for example using analytical “-omics” profiling approaches.
Related collections
Most cited references132
- Record: found
- Abstract: found
- Article: not found
Concise review: the surface markers and identity of human mesenchymal stem cells.
- Record: found
- Abstract: found
- Article: not found
Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives.

- Record: found
- Abstract: found
- Article: found