- Record: found
- Abstract: found
- Article: not found
Production of pharmaceutical proteins by transgenic animals
Abstract
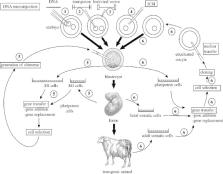
Proteins started being used as pharmaceuticals in the 1920s with insulin extracted from pig pancreas. In the early 1980s, human insulin was prepared in recombinant bacteria and it is now used by all patients suffering from diabetes. Several other proteins and particularly human growth hormone are also prepared from bacteria. This success was limited by the fact that bacteria cannot synthesize complex proteins such as monoclonal antibodies or coagulation blood factors which must be matured by post-translational modifications to be active or stable in vivo. These modifications include mainly folding, cleavage, subunit association, γ-carboxylation and glycosylation. They can be fully achieved only in mammalian cells which can be cultured in fermentors at an industrial scale or used in living animals. Several transgenic animal species can produce recombinant proteins but presently two systems started being implemented. The first is milk from farm transgenic mammals which has been studied for 20 years and which allowed a protein, human antithrombin III, to receive the agreement from EMEA (European Agency for the Evaluation of Medicinal Products) to be put on the market in 2006. The second system is chicken egg white which recently became more attractive after essential improvement of the methods used to generate transgenic birds. Two monoclonal antibodies and human interferon-β1a could be recovered from chicken egg white. A broad variety of recombinant proteins were produced experimentally by these systems and a few others. This includes monoclonal antibodies, vaccines, blood factors, hormones, growth factors, cytokines, enzymes, milk proteins, collagen, fibrinogen and others. Although these tools have not yet been optimized and are still being improved, a new era in the production of recombinant pharmaceutical proteins was initiated in 1987 and became a reality in 2006. In the present review, the efficiency of the different animal systems to produce pharmaceutical proteins are described and compared to others including plants and micro-organisms.
Résumé
Les protéines d’intérêt pharmaceutique ont commencé à être utilisées au cours des années 1920 avec l’insuline extraite des pancréas de porcs. Au début des années 1980, l’insuline humaine a commencé à être préparée à partir de bactéries recombinantes et désormais, tous les diabétiques utilisent cette hormone. Plusieurs autres protéines et notamment l’hormone de croissance humaine, ont été préparées à partir de bactéries recombinantes. Ces premiers succès ont rapidement montré la limite des bactéries qui sont incapables de synthétiser des protéines ayant une structure complexe comme les anticorps ou les facteurs de coagulation sanguine. En effet, pour être stables et actives in vivo, ces protéines doivent subir de multiples modifications post-traductionnelles. Les principales modifications sont le repliement, le clivage, l’association des sous-unités, la γ-carboxylation et la glycosylation. Elles ne se produisent complètement que dans des cellules de mammifères cultivées dans des fermenteurs à l’échelle industrielle ou appartenant à des animaux transgéniques. Plusieurs espèces d’animaux transgéniques peuvent produire des protéines recombinantes mais actuellement deux systèmes ont commencé à être exploités. Le premier est le lait des animaux de ferme transgéniques qui sont étudiés depuis 20 ans. Ce système a permis à une protéine, l’antithrombine III humaine, de recevoir l’autorisation de mise sur le marché par l’EMEA (European Agency for the Evaluation of Medicinal Products) en 2006. Le second système est le blanc d’œuf de poulets transgéniques qui est devenu récemment plus attractif après que les méthodes de préparation d’oiseaux transgéniques aient été améliorées. Deux anticorps monoclonaux et de l’interféron-β1a humain ont été obtenus dans le blanc d’œuf de poulets. Une grande variété de protéines recombinantes a été préparée à titre expérimental avec ces deux systèmes et quelques autres. Ces protéines comprennent des anticorps monoclonaux, des vaccins, des facteurs sanguins, des hormones, des facteurs de croissance, des cytokines, des enzymes, des protéines du lait, du collagène, du fribrinogène et d’autres encore. Bien que ces outils n’aient pas été optimisés et soient encore en cours d’amélioration, une nouvelle ère dans la production de protéines recombinantes pharmaceutiques a commencé en 1987 et est devenue une réalité en 2006. Dans cette revue, l’efficacité des différents systèmes animaux capables de produire des protéines pharmaceutiques sont décrits et comparés aux autres incluant les plantes et les microorganismes.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Germline transmission of genetically modified primordial germ cells.
- Record: found
- Abstract: found
- Article: not found
Production of cattle lacking prion protein.
- Record: found
- Abstract: found
- Article: not found
Plant cell cultures for the production of recombinant proteins.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.