- Record: found
- Abstract: found
- Article: found
Inflammatory processes in the prefrontal cortex induced by systemic immune challenge: Focusing on neurons
Read this article at
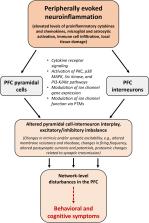
Abstract
Peripheral immune challenge induces neurobiological alterations in the brain and related neuropsychiatric symptoms both in humans and other mammals. One of the best known physiological effects of systemic inflammation is sickness behavior. However, in addition to this depression-like state, there are other cognitive outcomes of peripherally induced neuroinflammation that can be linked to the dysfunction of higher-order cortical areas, such as the prefrontal cortex (PFC). As the physiological activity of the PFC is largely based on the balanced interplay of excitatory pyramidal cells and inhibitory interneurons, it may be hypothesized that neuroinflammatory processes result in a shift of excitatory/inhibitory balance, which is a common hallmark of several neuropsychiatric conditions. Indeed, many data suggest that peripherally induced neuroinflammation is strongly associated with molecular and functional changes in PFC neurons leading to disturbances in their synaptic networks. Different experimental approaches may cause some incongruence in the reviewed data. However, it is commonly agreed that acute systemic inflammation leads to changes in the excitatory/inhibitory balance in the PFC by proinflammatory signaling at the brain borders and in the brain parenchyma. These cellular changes result in altered local and brain-wide network activity inducing disturbances in the top-down control of goal-directed behavior and cognition regulated by the PFC. Lipopolysaccharide (LPS)-treated rodents are the most widely used experimental models of peripherally induced neuroinflammation, so the majority of the reviewed data come from studies utilizing the LPS model. This may limit their general interpretation regarding the neuronal effects of peripheral immune activation. In addition, several biological variables (e.g., sex, age) can influence the PFC effects of systemic immune challenge, not only the nature and severity of immune activation. Therefore, it would be desirable to investigate inflammation-related neuronal changes in the PFC using other models of systemic inflammation as well, and to focus on the targeted fine-tuning of the affected cell types via common molecular mechanisms of the immune and nervous systems.
Highlights
-
•
Peripheral immune activation induces behavioral and cognitive symptoms.
-
•
PFC functions rely on the balanced interplay of excitatory and inhibitory neurons.
-
•
Systemic inflammation leads to molecular and functional changes in PFC neurons.
-
•
These neuronal changes result in excitatory/inhibitory imbalance in the PFC.
Related collections
Most cited references102
- Record: found
- Abstract: found
- Article: not found
Sex differences in immune responses
- Record: found
- Abstract: found
- Article: not found
Neuroinflammation: the devil is in the details.
- Record: found
- Abstract: found
- Article: not found