- Record: found
- Abstract: found
- Article: found
Acetaminophen impairs ferroptosis in the hippocampus of septic mice by regulating glutathione peroxidase 4 and ferroptosis suppressor protein 1 pathways
Read this article at
Abstract
Background
Neuronal ferroptosis is a major cause of cognitive impairment and mortality in patients with sepsis‐associated encephalopathy (SAE). A low dose of acetaminophen (APAP) in septic mice can prevent ferroptosis in the hippocampal tissue; however, the underlying mechanism is unknown. This study aimed to investigate the mechanism by which APAP reduces ferroptosis in the hippocampal tissues of septic mice.
Methods
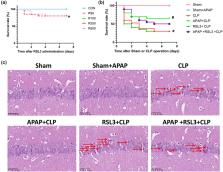
A mouse model of SAE was established, and the ferroptosis pathway inhibitors RSL3 and iFSP1+RSL3 were used in addition to APAP for the interventions, respectively. The 7‐day survival rate of the mice was recorded, and cognitive function was examined using the Morris water maze test. Hematoxylin and eosin staining was performed to observe hippocampal tissue damage. Hippocampal iron and malondialdehyde (MDA) were measured using chemical colorimetric methods. Immunofluorescence was used to detect the reactive oxygen species (ROS) content in hippocampal tissues.
Results
RSL3 reversed the efficacy of APAP on improving cognitive dysfunction in septic mice but did not obviously reverse the survival rate of mice enhanced by APAP. RSL3 aggravated APAP‐induced hippocampal tissue damage in mice attenuated by APAP. RSL3 inhibited glutathione peroxidase 4 (GPX4) expression and increased ferroptosis suppressor protein 1 (FSP1) and 4‐hydroxy‐2‐nonenal (4‐HNE) expression. RSL3 also reversed the effects of APAP in reducing iron, MDA, and ROS levels in the hippocampal tissues of septic mice. iFSP1+RSL3 further reversed the effect of APAP on ameliorating cognitive dysfunction in septic mice and successfully reversed the survival rate of mice enhanced by APAP. iFSP1+RSL3 aggravated APAP‐induced cerebral hippocampal damage. iFSP1+RSL3 inhibited both GPX4 and FSP1, further reversing the effect of APAP on the reduction in iron, 4‐HNE, ROS, and MDA levels in the cerebral hippocampus of mice with sepsis.
Abstract
Neuronal ferroptosis is a major cause of cognitive impairment and mortality in sepsis‐associated encephalopathy (SAE). In septic mice, a little dosage of acetaminophen (APAP) can prevent hippocampus tissue ferroptosis, however the mechanism is unknown. The goal of this study was to investigate the mechanism through which APAP reduces ferroptosis in septic mice hippocampus tissue.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease
- Record: found
- Abstract: found
- Article: not found
The CoQ oxidoreductase FSP1 acts in parallel to GPX4 to inhibit ferroptosis
- Record: found
- Abstract: not found
- Article: not found

