- Record: found
- Abstract: found
- Article: found
Robotic platform for microinjection into single cells in brain tissue
Read this article at
Abstract
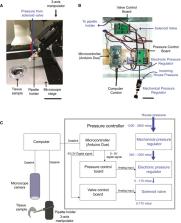
Microinjection into single cells in brain tissue is a powerful technique to study and manipulate neural stem cells. However, such microinjection requires expertise and is a low‐throughput process. We developed the “Autoinjector”, a robot that utilizes images from a microscope to guide a microinjection needle into tissue to deliver femtoliter volumes of liquids into single cells. The Autoinjector enables microinjection of hundreds of cells within a single organotypic slice, resulting in an overall yield that is an order of magnitude greater than manual microinjection. The Autoinjector successfully targets both apical progenitors ( APs) and newborn neurons in the embryonic mouse and human fetal telencephalon. We used the Autoinjector to systematically study gap‐junctional communication between neural progenitors in the embryonic mouse telencephalon and found that apical contact is a characteristic feature of the cells that are part of a gap junction‐coupled cluster. The throughput and versatility of the Autoinjector will render microinjection an accessible high‐performance single‐cell manipulation technique and will provide a powerful new platform for performing single‐cell analyses in tissue for bioengineering and biophysics applications.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
The molecular biology of memory storage: a dialogue between genes and synapses.
- Record: found
- Abstract: not found
- Article: not found
The cell biology of neurogenesis.
- Record: found
- Abstract: found
- Article: not found