- Record: found
- Abstract: found
- Article: not found
An ARF6-Exportin-5 Axis Delivers pre-miRNA Cargo to Tumor Microvesicles.
Abstract
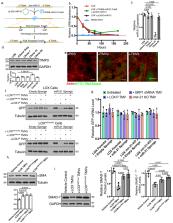
Tumor-derived microvesicles (TMVs) comprise a class of extracellular vesicles released from tumor cells that are now understood to facilitate communication between the tumor and the surrounding microenvironment. Despite their significance, the regulatory mechanisms governing the trafficking of bioactive cargos to TMVs at the cell surface remain poorly defined. Here we describe a molecular pathway for the delivery of microRNA (miRNA) cargo to nascent TMVs involving the dissociation of a pre-miRNA/Exportin-5 complex from Ran-GTP following nuclear export, and its subsequent transfer to a cytoplasmic shuttle comprised of ARF6-GTP and GRP1. As such, ARF6 activation increases pre-miRNA cargo contained within TMVs via a process that requires casein kinase 2-mediated phosphorylation of Ran-GAP1. Further, TMVs were found to contain pre-miRNA processing machinery including Dicer and Argonaute 2, which allow for cell-free pre-miRNA processing within shed vesicles. These findings offer cellular targets to block the loading and processing of pre-miRNAs within TMVs.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Large-scale mapping of human protein–protein interactions by mass spectrometry

- Record: found
- Abstract: found
- Article: found
Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types
- Record: found
- Abstract: found
- Article: not found
