- Record: found
- Abstract: found
- Article: found
Tuning DO:DM Ratios Modulates MHC Class II Immunopeptidomes
Read this article at
Abstract
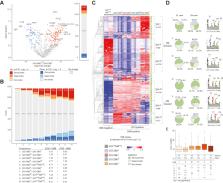
Major histocompatibility complex class II (MHC-II) antigen presentation underlies a wide range of immune responses in health and disease. However, how MHC-II antigen presentation is regulated by the peptide-loading catalyst HLA-DM (DM), its associated modulator, HLA-DO (DO), is incompletely understood. This is due largely to technical limitations: model antigen-presenting cell (APC) systems that express these MHC-II peptidome regulators at physiologically variable levels have not been described. Likewise, computational prediction tools that account for DO and DM activities are not presently available. To address these gaps, we created a panel of single MHC-II allele, HLA-DR4-expressing APC lines that cover a wide range of DO:DM ratio states. Using a combined immunopeptidomic and proteomic discovery strategy, we measured the effects DO:DM ratios have on peptide presentation by surveying over 10,000 unique DR4-presented peptides. The resulting data provide insight into peptide characteristics that influence their presentation with increasing DO:DM ratios. These include DM sensitivity, peptide abundance, binding affinity and motif, peptide length, and choice of binding register along the source protein. These findings have implications for designing improved HLA-II prediction algorithms and research strategies for dissecting the variety of functions that different APCs serve in the body.
Graphical Abstract
Highlights
In Brief
Immunopeptide presentation by MHC-II regulates adaptive immunity. The noncanonical MHC molecules HLA-DM and HLA-DO cooperatively regulate MHC-II function, and their relative abundances vary across APCs and cellular contexts. How this variation influences immunopeptide repertoires remains unclear. We addressed this by creating cell lines expressing HLA-DM and HLA-DO, spanning several relative abundances and measuring their immunopeptide repertoires. We found that immunopeptides clustered according to their presentation levels across different DO:DM ratios. Predicted MHC-II binding affinity substantially contributed to but did not fully account for these results.
Related collections
Most cited references65
- Record: found
- Abstract: not found
- Article: not found
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing
- Record: found
- Abstract: found
- Article: not found
Systematic and quantitative assessment of the ubiquitin-modified proteome.

- Record: found
- Abstract: found
- Article: found
