- Record: found
- Abstract: found
- Article: not found
Targeting nuclear RNA for in vivo correction of myotonic dystrophy
Abstract
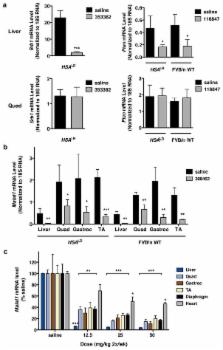
Antisense oligonucleotides (ASOs) hold promise for gene-specific knockdown in diseases that involve RNA or protein gain-of-function. In the hereditary degenerative disease myotonic dystrophy type 1 (DM1), transcripts from the mutant allele contain an expanded CUG repeat 1– 3 and are retained in the nucleus 4, 5 . The mutant RNA exerts a toxic gain-of-function 6 , making it an appropriate target for therapeutic ASOs. However, despite improvements in ASO chemistry and design, systemic use of ASOs is limited because uptake in many tissues, including skeletal and cardiac muscle, is not sufficient to silence target mRNAs 7, 8 . Here we show that nuclear-retained transcripts containing expanded CUG (CUG exp) repeats are extraordinarily sensitive to antisense silencing. In a transgenic mouse model of DM1, systemic administration of ASOs caused a rapid knockdown of CUG exp RNA in skeletal muscle, correcting the physiological, histopathologic, and transcriptomic features of the disease. The effect was sustained for up to one year after treatment was discontinued. Systemically administered ASOs were also effective for muscle knockdown of Malat-1, a long noncoding RNA (lncRNA) that is retained in the nucleus 9 . These results provide a general strategy to correct RNA gain-of-function and modulate the expression of expanded repeats, lncRNAs, and other transcripts with prolonged nuclear residence.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS.
- Record: found
- Abstract: not found
- Article: not found
What is principal component analysis?
- Record: found
- Abstract: found
- Article: not found
