- Record: found
- Abstract: found
- Article: not found
Notch Post-Translationally Regulates β-Catenin Protein in Stem and Progenitor Cells
Read this article at
Abstract
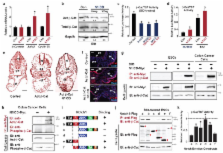
Cellular decisions of self-renewal or differentiation arise from integration and reciprocal titration of numerous regulatory networks. Notch and Wnt/β-Catenin signaling often intersect in stem and progenitor cells and regulate one another transcriptionally. The biological outcome of signaling through each pathway often depends on the context and timing as cells progress through stages of differentiation. Here, we show that membrane-bound Notch physically associates with unphosphorylated (active) β-Catenin in stem and colon cancer cells and negatively regulates post-translational accumulation of active β-Catenin protein. Notch-dependent regulation of β-Catenin protein did not require ligand-dependent membrane cleavage of Notch or the glycogen synthase kinase-3β-dependent activity of the β-catenin destruction complex. It did, however, require the endocytic adaptor protein, Numb, and lysosomal activity. This study reveals a previously unrecognized function of Notch in negatively titrating active β-Catenin protein levels in stem and progenitor cells.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Notch signaling controls multiple steps of pancreatic differentiation.
- Record: found
- Abstract: found
- Article: not found
Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells.
- Record: found
- Abstract: found
- Article: not found
