- Record: found
- Abstract: found
- Article: found
Developments and opportunities in continuous biopharmaceutical manufacturing
Read this article at
ABSTRACT
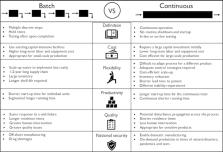
Today’s biologics manufacturing practices incur high costs to the drug makers, which can contribute to high prices for patients. Timely investment in the development and implementation of continuous biomanufacturing can increase the production of consistent-quality drugs at a lower cost and a faster pace, to meet growing demand. Efficient use of equipment, manufacturing footprint, and labor also offer the potential to improve drug accessibility. Although technological efforts enabling continuous biomanufacturing have commenced, challenges remain in the integration, monitoring, and control of traditionally segmented unit operations. Here, we discuss recent developments supporting the implementation of continuous biomanufacturing, along with their benefits.
Related collections
Most cited references166
- Record: found
- Abstract: found
- Article: not found
Antibody structure, instability, and formulation.
- Record: found
- Abstract: not found
- Article: not found
Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study

- Record: found
- Abstract: found
- Article: found
