- Record: found
- Abstract: found
- Article: found
VEGF-A modulates expression of inhibitory checkpoints on CD8 + T cells in tumors
Read this article at
Abstract
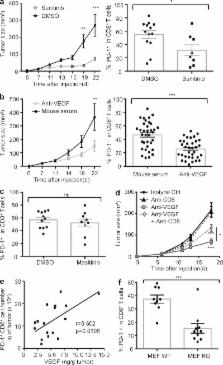
VEGF-A production in the tumor microenvironment enhances expression of PD-1 and other inhibitory checkpoints involved with CD8 + T cell exhaustion, which can be reversed with anti-VEGF/VEGFR treatment.
Abstract
Immune escape is a prerequisite for tumor development. To avoid the immune system, tumors develop different mechanisms, including T cell exhaustion, which is characterized by expression of immune inhibitory receptors, such as PD-1, CTLA-4, Tim-3, and a progressive loss of function. The recent development of therapies targeting PD-1 and CTLA-4 have raised great interest since they induced long-lasting objective responses in patients suffering from advanced metastatic tumors. However, the regulation of PD-1 expression, and thereby of exhaustion, is unclear. VEGF-A, a proangiogenic molecule produced by the tumors, plays a key role in the development of an immunosuppressive microenvironment. We report in the present work that VEGF-A produced in the tumor microenvironment enhances expression of PD-1 and other inhibitory checkpoints involved in CD8 + T cell exhaustion, which could be reverted by anti-angiogenic agents targeting VEGF-A–VEGFR. In view of these results, association of anti-angiogenic molecules with immunomodulators of inhibitory checkpoints may be of particular interest in VEGF-A-producing tumors.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies.
- Record: found
- Abstract: found
- Article: not found
Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients.
- Record: found
- Abstract: found
- Article: not found