- Record: found
- Abstract: found
- Article: not found
Models for oral uptake of nanoparticles in consumer products
Read this article at
Abstract
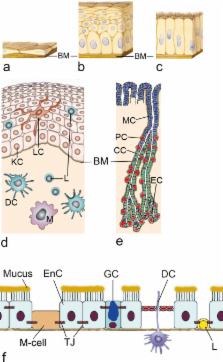
Presently, many consumer products contain nano-sized materials (NMs) to improve material properties, product quality and ease of use. NMs in food additives and in cosmetic articles (e.g., tooth paste) may be taken up by the oral route. As adverse effects of environmental nanoparticles, like ultrafine particles, have been reported, consumers worry about potential risks when using products containing NMs. The review focuses on metal and metal oxide NMs as common additives in tooth paste and in food industry and exposure by the oral route. Testing of NMs for oral exposure is very complex because differences in the diet, in mucus secretion and composition, in pH, in gastrointestinal transit time and in gastrointestinal flora influence NM uptake. Acellular (mucus, saliva) and epithelial layer of the orogastrointestinal barrier are described. Expected exposure doses, interaction of the NMs with mucus and permeation through the epithelium as well as in vivo data are mentioned. The role of in vitro models for the study of parameters relevant for ingested NMs is discussed.
Related collections
Most cited references104
- Record: found
- Abstract: found
- Article: not found
Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm.
- Record: found
- Abstract: found
- Article: not found
