- Record: found
- Abstract: found
- Article: found
Analysis of the Effect of Increased α2,3-Sialylation on RTK Activation in MKN45 Gastric Cancer Spheroids Treated with Crizotinib
Read this article at
Abstract
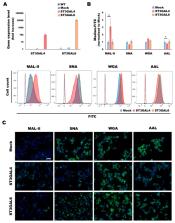
In the scenario of personalized medicine, targeted therapies are currently the focus of cancer drug development. These drugs can block the growth and spread of tumor cells by interfering with key molecules involved in malignancy, such as receptor tyrosine kinases (RTKs). MET and Recepteur d’Origine Nantais (RON), which are RTKs frequently overactivated in gastric cancer, are glycoprotein receptors whose activation have been shown to be modulated by the cellular glycosylation. In this work, we address the role of sialylation in gastric cancer therapy using an innovative 3D high-throughput cell culture methodology that mimics better the in vivo tumor features. We evaluate the response to targeted treatment of glycoengineered gastric cancer cell models overexpressing the sialyltransferases ST3GAL4 or ST3GAL6 by subjecting 3D spheroids to the tyrosine kinase inhibitor crizotinib. We show here that 3D spheroids of ST3GAL4 or ST3GAL6 overexpressing MKN45 gastric cancer cells are less affected by the inhibitor. In addition, we disclose a potential compensatory pathway via activation of the Insulin Receptor upon crizotinib treatment. Our results suggest that cell sialylation, in addition of being involved in tumor progression, could play a critical role in the response to tyrosine kinase inhibitors in gastric cancer.
Related collections
Most cited references46

- Record: found
- Abstract: found
- Article: found
Kinase-targeted cancer therapies: progress, challenges and future directions

- Record: found
- Abstract: found
- Article: found