- Record: found
- Abstract: found
- Article: found
Cell cycle dynamics and complement expression distinguishes mature haematopoietic subsets arising from hemogenic endothelium
Read this article at
ABSTRACT
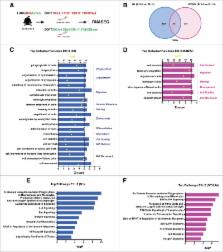
The emergence of haematopoietic stem and progenitor cells (HSPCs) from hemogenic endothelium results in the formation of sizeable HSPC clusters attached to the vascular wall. We evaluate the cell cycle and proliferation of HSPCs involved in cluster formation, as well as the molecular signatures from their initial appearance to the point when cluster cells are capable of adult engraftment (definitive HSCs). We uncover a non-clonal origin of HSPC clusters with differing cell cycle, migration, and cell signaling attributes. In addition, we find that the complement cascade is highly enriched in mature HSPC clusters, possibly delineating a new role for this pathway in engraftment.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Hematopoietic stem cells derive directly from aortic endothelium during development
- Record: found
- Abstract: found
- Article: not found
Blood stem cells emerge from aortic endothelium by a novel type of cell transition.
- Record: found
- Abstract: found
- Article: not found