- Record: found
- Abstract: found
- Article: found
The JAK1 Selective Inhibitor ABT 317 Blocks Signaling Through Interferon-γ and Common γ Chain Cytokine Receptors to Reverse Autoimmune Diabetes in NOD Mice
Read this article at
Abstract
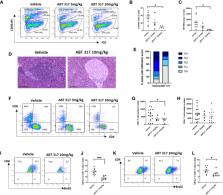
Cytokines that signal through the JAK-STAT pathway, such as interferon-γ (IFN-γ) and common γ chain cytokines, contribute to the destruction of insulin-secreting β cells by CD8 + T cells in type 1 diabetes (T1D). We previously showed that JAK1/JAK2 inhibitors reversed autoimmune insulitis in non-obese diabetic (NOD) mice and also blocked IFN-γ mediated MHC class I upregulation on β cells. Blocking interferons on their own does not prevent diabetes in knockout NOD mice, so we tested whether JAK inhibitor action on signaling downstream of common γ chain cytokines, including IL-2, IL-7 IL-15, and IL-21, may also affect the progression of diabetes in NOD mice. Common γ chain cytokines activate JAK1 and JAK3 to regulate T cell proliferation. We used a JAK1-selective inhibitor, ABT 317, to better understand the specific role of JAK1 signaling in autoimmune diabetes. ABT 317 reduced IL-21, IL-2, IL-15 and IL-7 signaling in T cells and IFN-γ signaling in β cells, but ABT 317 did not affect GM-CSF signaling in granulocytes. When given in vivo to NOD mice, ABT 317 reduced CD8 + T cell proliferation as well as the number of KLRG + effector and CD44 hiCD62L lo effector memory CD8 + T cells in spleen. ABT 317 also prevented MHC class I upregulation on β cells. Newly diagnosed diabetes was reversed in 94% NOD mice treated twice daily with ABT 317 while still on treatment at 40 days and 44% remained normoglycemic after a further 60 days from discontinuing the drug. Our results indicate that ABT 317 blocks common γ chain cytokines in lymphocytes and interferons in lymphocytes and β cells and are thus more effective against diabetes pathogenesis than IFN-γ receptor deficiency alone. Our studies suggest use of this class of drug for the treatment of type 1 diabetes.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Tofacitinib or adalimumab versus placebo in rheumatoid arthritis.
- Record: found
- Abstract: found
- Article: not found
JAK inhibition as a therapeutic strategy for immune and inflammatory diseases
- Record: found
- Abstract: found
- Article: not found