- Record: found
- Abstract: found
- Article: found
Effect of Adding Nano Size Silica on Setting Time and Porosity of Mineral Trioxide Aggregate
Read this article at
Abstract
Introduction:
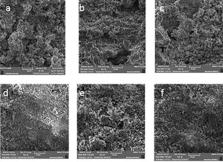
The aim of this study was to evaluate the effect of addition of nano-silica (SiO 2) to mineral trioxide aggregate (MTA) on its setting time and porosity.
Methods and Materials:
The concentration 8% of nano-silica were prepared and added to the MTA powder. After mixing with water the setting time and porosity were evaluated and compared with pure MTA. Statistical analysis was performed using the t-test. The level of significance was set at 0.001.
Results:
The mean setting time of MTA+8% nano-silica (9.8±0.78) was significantly lower than MTA (23.3±2.16) ( P<0.001). Also the mean porosity by imbibition method in MTA+8% nano-silica (23.49±0.48) was significantly higher than MTA (15.69±2.10) ( P<0.001). There was no significant difference in mean porosity by scanning electron microscope (SEM) method in MTA+8% nano-silica (31.26±10.73) and MTA (32.74±5.26) ( P>0.001).
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action.
- Record: found
- Abstract: found
- Article: not found
Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties.
- Record: found
- Abstract: found
- Article: not found