- Record: found
- Abstract: found
- Article: found
Exploring the Mediators that Promote Carotid Body Dysfunction in Type 2 Diabetes and Obesity Related Syndromes
Read this article at
Abstract
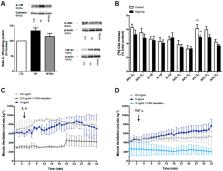
Carotid bodies (CBs) are peripheral chemoreceptors that sense changes in blood O 2, CO 2, and pH levels. Apart from ventilatory control, these organs are deeply involved in the homeostatic regulation of carbohydrates and lipid metabolism and inflammation. It has been described that CB dysfunction is involved in the genesis of metabolic diseases and that CB overactivation is present in animal models of metabolic disease and in prediabetes patients. Additionally, resection of the CB-sensitive nerve, the carotid sinus nerve (CSN), or CB ablation in animals prevents and reverses diet-induced insulin resistance and glucose intolerance as well as sympathoadrenal overactivity, meaning that the beneficial effects of decreasing CB activity on glucose homeostasis are modulated by target-related efferent sympathetic nerves, through a reflex initiated in the CBs. In agreement with our pre-clinical data, hyperbaric oxygen therapy, which reduces CB activity, improves glucose homeostasis in type 2 diabetes patients. Insulin, leptin, and pro-inflammatory cytokines activate the CB. In this manuscript, we review in a concise manner the putative pathways linking CB chemoreceptor deregulation with the pathogenesis of metabolic diseases and discuss and present new data that highlight the roles of hyperinsulinemia, hyperleptinemia, and chronic inflammation as major factors contributing to CB dysfunction in metabolic disorders.
Related collections
Most cited references139
- Record: found
- Abstract: found
- Article: not found
Adipose tissue dysfunction in obesity, diabetes, and vascular diseases.
- Record: found
- Abstract: found
- Article: not found
Interleukin-6-deficient mice develop mature-onset obesity.

- Record: found
- Abstract: found
- Article: found