- Record: found
- Abstract: found
- Article: found
Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice
Read this article at
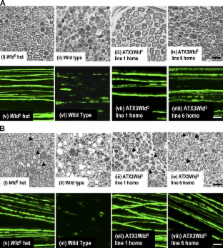
Abstract
The slow Wallerian degeneration (Wld S) protein protects injured axons from degeneration. This unusual chimeric protein fuses a 70–amino acid N-terminal sequence from the Ube4b multiubiquitination factor with the nicotinamide adenine dinucleotide–synthesizing enzyme nicotinamide mononucleotide adenylyl transferase 1. The requirement for these components and the mechanism of Wld S-mediated neuroprotection remain highly controversial. The Ube4b domain is necessary for the protective phenotype in mice, but precisely which sequence is essential and why are unclear. Binding to the AAA adenosine triphosphatase valosin-containing protein (VCP)/p97 is the only known biochemical property of the Ube4b domain. Using an in vivo approach, we show that removing the VCP-binding sequence abolishes axon protection. Replacing the Wld S VCP-binding domain with an alternative ataxin-3–derived VCP-binding sequence restores its protective function. Enzyme-dead Wld S is unable to delay Wallerian degeneration in mice. Thus, neither domain is effective without the function of the other. Wld S requires both of its components to protect axons from degeneration.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Axon degeneration mechanisms: commonality amid diversity.
- Record: found
- Abstract: found
- Article: not found
Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene.
- Record: found
- Abstract: not found
- Article: not found