- Record: found
- Abstract: found
- Article: found
Antidromic Spike Propagation and Dissimilar Expression of P2X, 5-HT, and TRPV1 Channels in Peripheral vs. Central Sensory Axons in Meninges
Read this article at
Abstract
Background: The terminal branches of the trigeminal nerve in meninges are supposed to be the origin site of migraine pain. The main function of these peripheral sensory axons is the initiation and propagation of spikes in the orthodromic direction to the second order neurons in the brainstem. The stimulation of the trigeminal ganglion induces the release of the neuropeptide CGRP in meninges suggesting the antidromic propagation of excitation in these fibers. However, the direct evidence on antidromic spike traveling in meningeal afferents is missing.
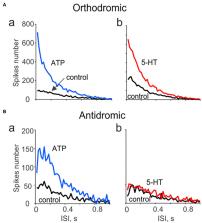
Methods: By recording of spikes from peripheral or central parts of the trigeminal nerve in rat meninges, we explored their functional activity and tested the expression of ATP-, serotonin-, and capsaicin-gated receptors in the distal vs. proximal parts of these nerves.
Results: We show the significant antidromic propagation of spontaneous spikes in meningeal nerves which was, however, less intense than the orthodromic nociceptive traffic due to higher number of active fibers in the latter. Application of ATP, serotonin and capsaicin induced a high frequency nociceptive firing in peripheral processes while, in central parts, only ATP and capsaicin were effective. Disconnection of nerve from trigeminal ganglion dramatically reduced the tonic antidromic activity and attenuated the excitatory action of ATP.
Conclusion: Our data indicate the bidirectional nociceptive traffic and dissimilar expression of P2X, 5-HT and TRPV1 receptors in proximal vs. distal parts of meningeal afferents, which is important for understanding the peripheral mechanisms of migraine pain.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release.
- Record: found
- Abstract: found
- Article: not found