- Record: found
- Abstract: found
- Article: found
Randomized phase 2 study of gemcitabine and cisplatin with or without vitamin supplementation in patients with advanced esophagogastric cancer
Read this article at
Abstract
Purpose
Preclinical research and prior clinical observations demonstrated reduced toxicity and suggested enhanced efficacy of cisplatin due to folic acid and vitamin B12 suppletion. In this randomized phase 2 trial, we evaluated the addition of folic acid and vitamin B12 to first-line palliative cisplatin and gemcitabine in patients with advanced esophagogastric cancer (AEGC).
Methods
Patients with AEGC were randomized to gemcitabine 1250 mg/m 2 (i.v. days 1, 8) and cisplatin 80 mg/m 2 (i.v. day 1) q 3 weeks with or without folic acid (450 µg/day p.o.) and vitamin B12 (1000 µg i.m. q 9 weeks). The primary endpoint was response rate (RR). Secondary endpoints included overall survival (OS), time to progression (TTP), toxicity, and exploratory biomarker analyses. Cisplatin sensitivity and intracellular platinum levels were determined in adenocarcinoma cell lines cultured under high and low folate conditions in vitro.
Results
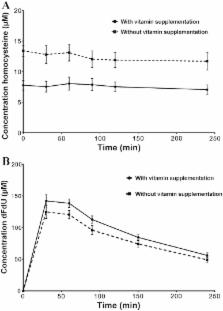
Adenocarcinoma cells cultured in medium with high folate levels were more sensitive to cisplatin and this was associated with increased intracellular platinum levels. In the randomized phase 2 clinical trial, which ran from October 2004 to September 2013, treatment was initiated in 78 of 82 randomized pts, 39 in each study arm. The RR was similar; 42.1% for supplemented patients vs. 32.4% for unsupplemented patients; p = 0.4. Median OS and TTP were 10.0 and 5.9 months for supplemented vs. 7.7 and 5.4 months for unsupplemented patients (OS, p = 0.9; TTP, p = 0.9). Plasma homocysteine was lower in the supplemented group [ n = 20, 6.9 ± 1.6 (mean ± standard error of mean, SEM) µM; vs. 12.5 ± 4.0 µM; p < 0.001]. There was no significant difference in the C max of gemcitabine and cisplatin in the two treatment groups.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma.
- Record: found
- Abstract: found
- Article: not found
Capecitabine and oxaliplatin for advanced esophagogastric cancer.
- Record: found
- Abstract: found
- Article: not found