- Record: found
- Abstract: found
- Article: found
Gangliosides in Podocyte Biology and Disease
Read this article at
Abstract
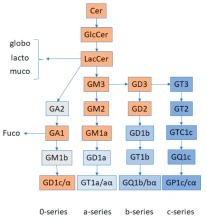
Gangliosides constitute a subgroup of glycosphingolipids characterized by the presence of sialic acid residues in their structure. As constituents of cellular membranes, in particular of raft microdomains, they exert multiple functions, some of them capital in cell homeostasis. Their presence in cells is tightly regulated by a balanced expression and function of the enzymes responsible for their biosynthesis, ganglioside synthases, and their degradation, glycosidases. The dysregulation of their abundance results in rare and common diseases. In this review, we make a point on the relevance of gangliosides and some of their metabolic precursors, such as ceramides, in the function of podocytes, the main cellular component of the glomerular filtration barrier, as well as their implications in podocytopathies. The results presented in this review suggest the pertinence of clinical lipidomic studies targeting these metabolites.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Revitalizing membrane rafts: new tools and insights.
- Record: found
- Abstract: found
- Article: not found
Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis.
- Record: found
- Abstract: found
- Article: not found