- Record: found
- Abstract: found
- Article: found
The roles of mutated SWI/SNF complexes in the initiation and development of hepatocellular carcinoma and its regulatory effect on the immune system: A review
Read this article at
Abstract
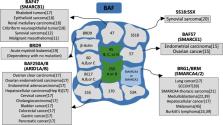
Hepatocellular carcinoma (HCC) is a primary liver malignancy with a high global prevalence and a dismal prognosis. Studies are urgently needed to examine the molecular pathogenesis and biological characteristics of HCC. Chromatin remodelling, an integral component of the DNA damage response, protects against DNA damage‐induced genome instability and tumorigenesis by triggering the signalling events that activate the interconnected DNA repair pathways. The SWI/SNF complexes are one of the most extensively investigated adenosine triphosphate‐dependent chromatin remodelling complexes, and mutations in genes encoding SWI/SNF subunits are frequently observed in various human cancers, including HCC. The mutated SWI/SNF complex subunits exert dual functions by accelerating or inhibiting HCC initiation and progression. Furthermore, the abnormal SWI/SNF complexes influence the transcription of interferon‐stimulated genes, as well as the differentiation, activation and recruitment of several immune cell types. In addition, they exhibit synergistic effects with immune checkpoint inhibitors in the treatment of diverse tumour types. Therefore, understanding the mutations and deficiencies of the SMI/SNF complexes, together with the associated functional mechanisms, may provide a novel strategy to treat HCC through targeting the related genes or modulating the tumour microenvironment.
Related collections
Most cited references116
- Record: found
- Abstract: found
- Article: not found
A unique chromatin signature uncovers early developmental enhancers in humans.
- Record: found
- Abstract: found
- Article: not found
Chromatin remodelling during development.
- Record: found
- Abstract: found
- Article: not found