- Record: found
- Abstract: found
- Article: found
CCL25/CCR9 interaction promotes the malignant behavior of salivary adenoid cystic carcinoma via the PI3K/AKT signaling pathway
Read this article at
Abstract
Background
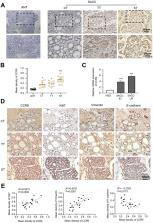
CC chemokine receptor 9 (CCR9), an organ-specific chemokine receptor, interacts with its exclusive ligand CCL25 to promote tumor proliferation and metastasis. However, the effect of CCR9 on salivary adenoid cystic carcinoma (SACC) malignant behavior remains unknown. This study aimed to investigate the specific molecular mechanism by which CCR9/CCL25 modulates malignant progression in SACC.
Methods
Immunohistochemistry staining and RT–qPCR analyses were performed to detect the correlation of CCR9 expression and tumor progression-associated markers in SACC. In vitro, SACC cell proliferation and apoptosis were evaluated using Cell Counting Kit-8 and colon formation, and cell migration and invasion were detected by wound healing and transwell assays. Vercirnon was used as an inhibitor of CCR9, and LY294002 was used as an inhibitor of the PI3K/AKT pathway in this study. Western blot and RT–qPCR assays were carried out to measure the downstream factors of the interaction of CCL25 and CCR9. The effect of CCL25 on the development of SACC in vivo was examined by a xenograft tumor model in nude mice following CCL25, Vercirnon and LY294002 treatment.
Results
CCR9 was highly expressed in SACC compared with adjacent salivary gland tissues, and its level was associated with tumor proliferation and metastases. CCL25 enhanced cell proliferation, migration, and invasion through its interaction with CCR9 and exerted an antiapoptotic effect on SACC cells. Targeting CCR9 via Vercirnon significantly reduced the phosphorylation level of AKT induced by CCL25. CCL25/CCR9 could activate its downstream factors through the PI3K/AKT signaling pathway, such as cyclin D1, BCL2 and SLUG, thus promoting SACC cell proliferation, antiapoptosis, invasion and metastasis. The in vivo data from the xenograft mouse models further proved that CCL25 administration promoted malignant tumor progression by activating the PI3K/AKT pathway.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
EMT Transition States during Tumor Progression and Metastasis
- Record: found
- Abstract: found
- Article: not found
The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism
- Record: found
- Abstract: found
- Article: not found