- Record: found
- Abstract: found
- Article: found
Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4
Read this article at
Abstract
Background
Acquisition of the M1 or M2 phenotypes by microglia has been shown to occur during the development of pathological conditions, with M1 activation being widely involved in neurotoxicity in relation with the anatomical localization and the reactivity of subtypes of microglia cells. On the contrary, little is known on the ability of microglia to undergo M2 polarization by interleukin-4 (IL4), the typical M2a polarization signal for peripheral macrophages.
Methods
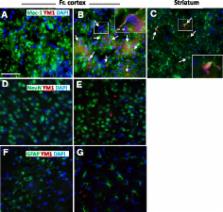
Recombinant mouse IL4 was injected in the third cerebral ventricle of mice to induce brain alternative polarization. The mRNA levels of Fizz1, Arg1, and Ym1 genes, known to be up-regulated by IL4 in peripheral macrophages, together with additional polarization markers, were evaluated in the striatum and frontal cortex at different time intervals after central administration of IL4; in parallel, M2a protein expression was evaluated in tissue extracts and at the cellular level.
Results
Our results show that the potency and temporal profile of IL4-mediated M2a gene induction vary depending on the gene analyzed and according to the specific brain area analyzed, with the striatum showing a reduced M2a response compared with the frontal cortex, as further substantiated by assays of polarization protein levels. Of notice, Fizz1 mRNA induction reached 100-fold level, underscoring the potency of this specific IL4 signaling pathway in the brain. In addition, immunochemistry assays demonstrated the localization of the M2 response specifically to microglia cells and, more interestingly, the existence of a subpopulation of microglia cells amenable to undergoing M2a polarization in the healthy mouse brain.
Conclusions
These results show that the responsiveness of brain macrophages to centrally administered IL4 may vary depending on the gene and brain area analyzed, and that M2a polarization can be ascribed to a subpopulation of IL4-responsive microglia cells. The biochemical pathways that enable microglia to undergo M2a activation represent key aspects for understanding the physiopathology of neuroinflammation and for developing novel therapeutic and diagnostic agents.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration.
- Record: found
- Abstract: found
- Article: not found
Heterogeneity of Microglial Activation in the Innate Immune Response in the Brain
- Record: found
- Abstract: found
- Article: not found