- Record: found
- Abstract: found
- Article: found
Cytotoxic lanthanum oxide nanoparticles sensitize glioblastoma cells to radiation therapy and temozolomide: an in vitro rationale for translational studies
Read this article at
Abstract
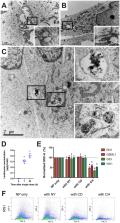
Glioblastoma (GBM) is a malignant brain tumour with a dismal prognosis, despite best treatment by surgical resection, radiation therapy (RT) and chemotherapy with temozolomide (TMZ). Nanoparticle (NP) therapy is an emerging consideration due to the ability of NPs to be formulated and cross the blood brain barrier. Lanthanum oxide (La 2O 3) NPs are therapeutically advantageous due to the unique chemical properties of lanthanum making it cytotoxic to cancers, and able to enhance existing anti-cancer treatments. However, La 2O 3 NPs have yet to be thoroughly investigated in brain tumors. We show that these NPs can reach the brain after venous injection, penetrate into GBM cells via endocytosis, dissociate to be cytotoxic, and enhance the therapeutic effects of RT and TMZ. The mechanisms of cell death by La 2O 3 NPs were found to be multifaceted. Increasing NP concentration was correlated to increased intrinsic and extrinsic apoptosis pathway markers in a radical oxygen species (ROS)-dependent manner, as well as involving direct DNA damage and autophagic pathways within GBM patient-derived cell lines. NP interactions to sensitize GBM to RT and TMZ were shown to involve these pathways by enhancing ROS and apoptotic mechanisms. We therefore demonstrate the therapeutic potential of La 2O 3 NPs to treat GBM cells in vitro, and encourage translational exploration in the future.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma
- Record: found
- Abstract: found
- Article: not found
Role of reactive oxygen species (ROS) in apoptosis induction.
- Record: found
- Abstract: not found
- Article: not found