- Record: found
- Abstract: found
- Article: found
Atg8 family LC3/GABARAP proteins are crucial for autophagosome–lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation
Read this article at
Abstract
Current autophagy models suggest that Atg8 family LC3/GABARAP proteins are essential mediators of autophagosome biogenesis. Nguyen et al. exploit CRISPR/Cas9-generated knockouts of the LC3 or GABARAP subfamilies, or both subfamilies, to show that Atg8s are dispensable for autophagosome biogenesis but essential for autophagosome–lysosome fusion.
Abstract
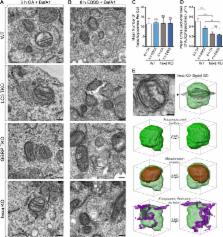
Members of the Atg8 family of proteins are conjugated to autophagosomal membranes, where they have been proposed to drive autophagosome formation and selective sequestration of cargo. In mammals, the Atg8 family consists of six members divided into the LC3 and GABARAP subfamilies. To define Atg8 function, we used genome editing to generate knockouts of the LC3 and GABARAP subfamilies as well as all six Atg8 family members in HeLa cells. We show that Atg8s are dispensable for autophagosome formation and selective engulfment of mitochondria, but essential for autophagosome–lysosome fusion. We find that the GABARAP subfamily promotes PLEKHM1 recruitment and governs autophagosome–lysosome fusion, whereas the LC3 subfamily plays a less prominent role in these processes. Although neither GABARAPs nor LC3s are required for autophagosome biogenesis, loss of all Atg8s yields smaller autophagosomes and a slowed initial rate of autophagosome formation. Our results clarify the essential function of the Atg8 family and identify GABARAP subfamily members as primary contributors to PINK1/Parkin mitophagy and starvation autophagy.
Related collections
Most cited references29

- Record: found
- Abstract: found
- Article: found
Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum
- Record: found
- Abstract: found
- Article: not found
A ubiquitin-like system mediates protein lipidation.
- Record: found
- Abstract: found
- Article: not found