- Record: found
- Abstract: found
- Article: not found
Ca 2+-induced Ca 2+ Release in Chromaffin Cells Seen from inside the ER with Targeted Aequorin
Read this article at
Abstract
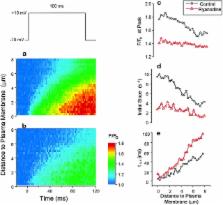
The presence and physiological role of Ca 2+-induced Ca 2+ release (CICR) in nonmuscle excitable cells has been investigated only indirectly through measurements of cytosolic [Ca 2+] ([Ca 2+] c). Using targeted aequorin, we have directly monitored [Ca 2+] changes inside the ER ([Ca 2+] ER) in bovine adrenal chromaffin cells. Ca 2+ entry induced by cell depolarization triggered a transient Ca 2+ release from the ER that was highly dependent on [Ca 2+] ER and sensitized by low concentrations of caffeine. Caffeine-induced Ca 2+ release was quantal in nature due to modulation by [Ca 2+] ER. Whereas caffeine released essentially all the Ca 2+ from the ER, inositol 1,4,5-trisphosphate (InsP 3)- producing agonists released only 60–80%. Both InsP 3 and caffeine emptied completely the ER in digitonin-permeabilized cells whereas cyclic ADP-ribose had no effect. Ryanodine induced permanent emptying of the Ca 2+ stores in a use-dependent manner after activation by caffeine. Fast confocal [Ca 2+] c measurements showed that the wave of [Ca 2+] c induced by 100-ms depolarizing pulses in voltage-clamped cells was delayed and reduced in intensity in ryanodine-treated cells. Our results indicate that the ER of chromaffin cells behaves mostly as a single homogeneous thapsigargin-sensitive Ca 2+ pool that can release Ca 2+ both via InsP 3 receptors or CICR.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches.
- Record: found
- Abstract: found
- Article: not found
Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria.
- Record: found
- Abstract: not found
- Article: not found
