- Record: found
- Abstract: found
- Article: found
Real-Time Fluorescence Loop Mediated Isothermal Amplification for the Diagnosis of Malaria
Read this article at
Abstract
Background
Molecular diagnostic methods can complement existing tools to improve the diagnosis of malaria. However, they require good laboratory infrastructure thereby restricting their use to reference laboratories and research studies. Therefore, adopting molecular tools for routine use in malaria endemic countries will require simpler molecular platforms. The recently developed loop-mediated isothermal amplification (LAMP) method is relatively simple and can be improved for better use in endemic countries. In this study, we attempted to improve this method for malaria diagnosis by using a simple and portable device capable of performing both the amplification and detection (by fluorescence) of LAMP in one platform. We refer to this as the RealAmp method.
Methodology and Significant Findings
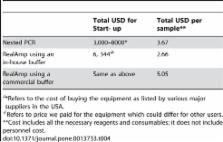
Published genus-specific primers were used to test the utility of this method. DNA derived from different species of malaria parasites was used for the initial characterization. Clinical samples of P. falciparum were used to determine the sensitivity and specificity of this system compared to microscopy and a nested PCR method. Additionally, directly boiled parasite preparations were compared with a conventional DNA isolation method. The RealAmp method was found to be simple and allowed real-time detection of DNA amplification. The time to amplification varied but was generally less than 60 minutes. All human-infecting Plasmodium species were detected. The sensitivity and specificity of RealAmp in detecting P. falciparum was 96.7% and 91.7% respectively, compared to microscopy and 98.9% and 100% respectively, compared to a standard nested PCR method. In addition, this method consistently detected P. falciparum from directly boiled blood samples.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies.
- Record: found
- Abstract: found
- Article: not found
Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis.
- Record: found
- Abstract: found
- Article: not found