- Record: found
- Abstract: found
- Article: found
Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe
Read this article at
Abstract
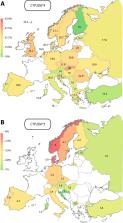
CYP2C19 and CYP2D6 are important drug-metabolizing enzymes that are involved in the metabolism of around 30% of all medications. Importantly, the corresponding genes are highly polymorphic and these genetic differences contribute to interindividual and interethnic differences in drug pharmacokinetics, response, and toxicity. In this study we systematically analyzed the frequency distribution of clinically relevant CYP2C19 and CYP2D6 alleles across Europe based on data from 82,791 healthy individuals extracted from 79 original publications and, for the first time, provide allele confidence intervals for the general population. We found that frequencies of CYP2D6 gene duplications showed a clear South-East to North-West gradient ranging from <1% in Sweden and Denmark to 6% in Greece and Turkey. In contrast, an inverse distribution was observed for the loss-of-function alleles CYP2D6*4 and CYP2D6*5. Similarly, frequencies of the inactive CYP2C19*2 allele were graded from North-West to South-East Europe. In important contrast to previous work we found that the increased activity allele CYP2C19*17 was most prevalent in Central Europe (25–33%) with lower prevalence in Mediterranean-South Europeans (11–24%). In summary, we provide a detailed European map of common CYP2C19 and CYP2D6 variants and find that frequencies of the most clinically relevant alleles are geographically graded reflective of Europe’s migratory history. These findings emphasize the importance of generating pharmacogenomic data sets with high spatial resolution to improve precision public health across Europe.
Related collections
Most cited references32

- Record: found
- Abstract: found
- Article: found
Worldwide Distribution of Cytochrome P450 Alleles: A Meta‐analysis of Population‐scale Sequencing Projects
- Record: found
- Abstract: found
- Article: not found
A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants.
- Record: found
- Abstract: found
- Article: not found